Ever noticed how red cabbage can transform into a vibrant blue hue? This captivating color change isn't magic, but fascinating science! It's all about the pH levels – the measure of acidity or alkalinity – of the substances it comes into contact with. This natural pH indicator has been captivating curious minds for centuries, and we're diving deep into the what, why, and how of this colorful phenomenon.
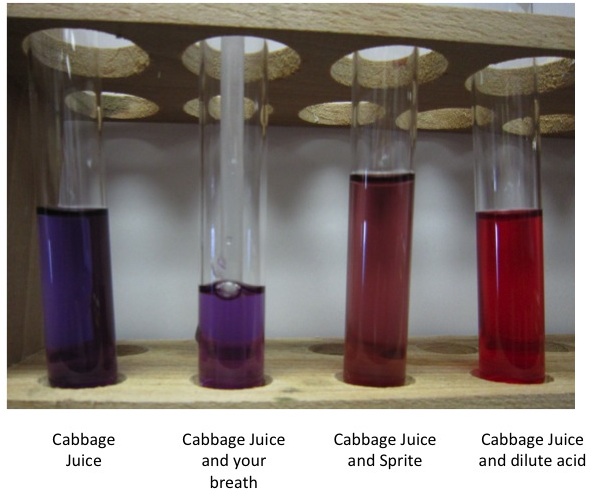
Red cabbage contains a pigment called anthocyanin, a natural pH indicator. When exposed to an alkaline substance (a base), the anthocyanin molecules change shape, resulting in a shift from red to blue or even green. Conversely, acidic substances maintain the cabbage's reddish-purple color. This dramatic color shift makes red cabbage juice a powerful tool for exploring acids and bases, both in the kitchen and the lab.
The color-changing properties of red cabbage have been recognized for centuries. Early chemists and scientists used red cabbage extracts to determine the acidity or alkalinity of different solutions, long before modern pH meters were invented. Today, this simple yet effective method continues to be used in educational settings and home experiments, showcasing the power of natural indicators.
The color change in red cabbage is more than just a cool trick. Understanding how different substances affect the color of the cabbage juice can provide valuable insights into the nature of acids and bases. This knowledge is not only essential in chemistry but also has practical applications in cooking, gardening, and even environmental testing.
Imagine using red cabbage juice as a natural food coloring! By adjusting the pH of a dish with ingredients like lemon juice (acidic) or baking soda (alkaline), you can manipulate the color of your creations. This opens up a world of possibilities for vibrant and naturally colored foods, adding an element of fun and excitement to cooking.
The shift of red cabbage to shades of blue indicates an alkaline environment. You can test this by adding baking soda to chopped red cabbage. The resulting blue color confirms the alkaline nature of the baking soda.
One benefit of understanding the red cabbage color change is its use as a natural pH indicator in home experiments. Another advantage is its application in creating naturally colored foods. Lastly, it promotes a deeper understanding of acids and bases and their interactions.
Create your own pH indicator! Finely chop red cabbage and simmer it in water. Strain the resulting purple liquid and use it to test the pH of various household substances like lemon juice, vinegar, and baking soda. Observe the color changes and identify the acidic and alkaline substances.
Advantages and Disadvantages of Using Red Cabbage as a pH Indicator
| Advantages | Disadvantages |
|---|---|
| Easy to obtain and prepare | Not as precise as a digital pH meter |
| Natural and safe to use | Color changes can be subjective |
| Cost-effective for simple experiments | Susceptible to temperature changes |
Several challenges can arise when using red cabbage indicator, such as difficulty in distinguishing subtle color changes. A solution is to create a color chart using known pH solutions for comparison.
FAQ: What makes red cabbage turn blue? How can I use red cabbage as a pH indicator? Can I use other vegetables for similar results? Why does the color change happen? Is the color change reversible? What are some common household acids and bases to test? What other natural pH indicators exist? How can I make my red cabbage indicator last longer?
Answers can vary but typically involve explaining the role of anthocyanins and pH levels.
A tip for using red cabbage as an indicator is to ensure the cabbage is fresh for vibrant color changes.
In conclusion, the color transformation of red cabbage from its characteristic purplish-red to various shades of blue, even green, provides a compelling and accessible demonstration of the fascinating world of pH and chemical reactions. This natural phenomenon, driven by the pH-sensitive anthocyanin pigments, has not only captivated scientific curiosity for centuries but also offers valuable practical applications. From educational experiments to creative culinary endeavors, the vibrant color changes of red cabbage serve as a reminder of the hidden wonders present in everyday ingredients. The ability to easily visualize pH levels through this natural indicator allows for a deeper understanding of acid-base chemistry and its relevance in our daily lives. As we have explored, the color change in red cabbage is not just a simple chemical reaction, but a window into the intricate interplay of molecules and their response to their environment. By understanding this process, we can not only appreciate the beauty of nature's chemistry but also harness its potential for practical use, fostering a greater appreciation for the science that surrounds us.
1 red cabbage many colours Experiment with kiddos Cabbage Juice Red - Trees By Bike
Red cabbage dye and pH indicator - Trees By Bike
Red Cabbage AcidBase Experiment - Trees By Bike
FAA closes investigation into Blue Origins New Shepard rocket failure - Trees By Bike
Cabbage Juice pH Chemistry Lab - Trees By Bike
Red Cabbage Blue Mtn - Trees By Bike
science chemistry acid base cabbage indicator - Trees By Bike
Red Cabbage as a pH Indicator - Trees By Bike
red cabbage turns blue - Trees By Bike
red cabbage turns blue - Trees By Bike
Acid base indicator lab Acids and Base Indicator childhealthpolicy - Trees By Bike
red cabbage turns blue - Trees By Bike
science chemistry acid base cabbage indicator - Trees By Bike
AP Chemistry Lab 11 Bromothymol Blue As A PH Indicator 53 OFF - Trees By Bike
Solved Blue litmus paper is converted into red in solution of - Trees By Bike