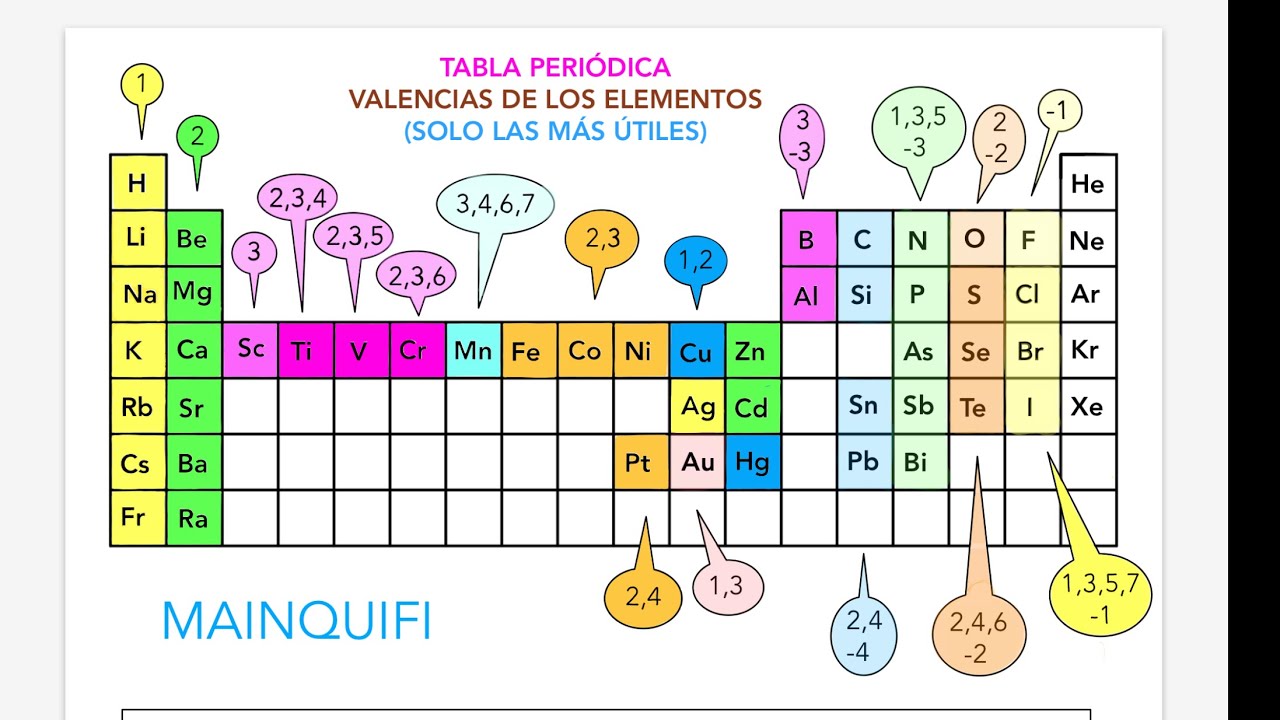
Imagine a vast library filled with the building blocks of our universe. Each element meticulously categorized, its properties laid bare. This, in essence, is the power of the periodic table – or as it's known in Spanish, the "tabla periodica." But what if we told you this intricate chart holds even more secrets, encoded within the "valencias" (valences) of each element?
The periodic table isn't just a static arrangement of elements; it's a dynamic tool that helps us understand how atoms interact, bond, and form the incredible diversity of molecules that make up our world. At the heart of this interaction lies the concept of valence – the combining power of an atom, dictated by the number of electrons it can gain, lose, or share to achieve a stable electronic configuration.
Delving into the "tabla periodica con todas las valencias" unveils a deeper layer of chemical understanding. It's like finding the instruction manual for building molecules! By knowing the valences, we can predict how elements will react, the types of bonds they will form, and ultimately, the properties of the resulting compounds.
Think of sodium (Na) with its single outer electron, eager to be donated, and chlorine (Cl) with its almost-full outer shell, yearning for one more electron to complete the set. Their valences, +1 for sodium and -1 for chlorine, practically scream "perfect match!" And indeed, they react explosively to form sodium chloride (NaCl) – the familiar table salt. This simple example illustrates the predictive power hidden within the valences on the periodic table.
Mastering the "tabla periodica con todas las valencias" empowers us to decode the language of chemistry. From predicting the formulas of complex compounds to understanding the behavior of elements in chemical reactions, the valence holds the key. It's a journey into the heart of matter, revealing the elegant rules that govern the interactions of the building blocks of our universe.
While the concept of "valencia" is deeply intertwined with the periodic table, it's essential to clarify that most periodic tables don't explicitly list all possible valences for each element directly on the chart. This is because valency can vary depending on the specific chemical environment and the atoms involved in bonding.
However, the periodic table's organization provides crucial clues about typical valences. Elements within the same group (vertical column) tend to exhibit similar valences due to having the same number of valence electrons—those crucial electrons in the outermost shell that participate in bonding. For example, Group 1 elements (alkali metals) generally have a valence of +1, readily losing one electron to achieve a stable configuration.
To truly unlock the full potential of the "tabla periodica con todas las valencias," it's crucial to delve deeper into understanding periodic trends, electron configurations, and different types of chemical bonding. Armed with this knowledge, the periodic table transforms from a simple chart into a powerful tool for predicting and explaining the fascinating world of chemical interactions.
Enfatizar artillería Ventana mundial todas las valencias de la tabla - Trees By Bike
Tabla periódica de los elementos: grupos y valencias - Trees By Bike
Numeros de Valencias de Los Elementos Quimicos - Trees By Bike
Tabla Periodica Y Sus Valencia's - Trees By Bike
Tabla Periódica Completa Con Nombres Y Valencias: ¡Domina La Química! - Trees By Bike
Tabla Periodica Actualizada Completa Con Sus Valencias Decoration - Trees By Bike
tabla periodica con valencias ciencias 2 186 e s o - Trees By Bike
Resultado de imagen para tabla periodica completa con numeros de - Trees By Bike
Tabla Periodica Valencias #tablaperiodica #TablaPeriodicaCompleta # - Trees By Bike
Formulación Inorgánica La tabla periódica y las valencias - Trees By Bike
grado costilla fregar juegos para aprender las valencias de la tabla - Trees By Bike
Tabla Periodica Con Valencias - Trees By Bike
Tabla periodica completa con valencias - Trees By Bike
Tabla Periódica Pdf - Trees By Bike
Tabla Periodica De Los Elementos Quimicos Actualizada Pdf Tabla - Trees By Bike